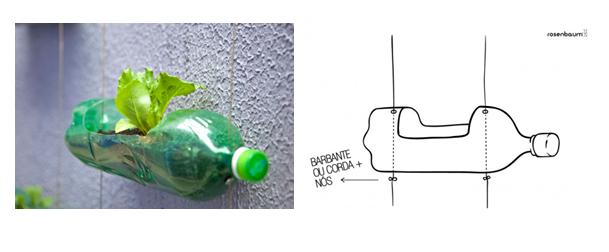
Huerto Vertical en casa con botellas de plástico. #EcoIdeas #Reciclaje #Jardín #medioambiente | Jardim vertical diy, Jardim de legumes, Hortas verticais

Cómo hacer un mini huerto con botellas de plástico | Upcycled planter, Plastic milk bottles, Flower pots